
I think this is when white light is used that you get an Absorption Spectra. All the colors of the Absorption Spectra do make it kind of confusing. Absorption occurs when electrons absorb photons which causes them to gain energy and jump to.
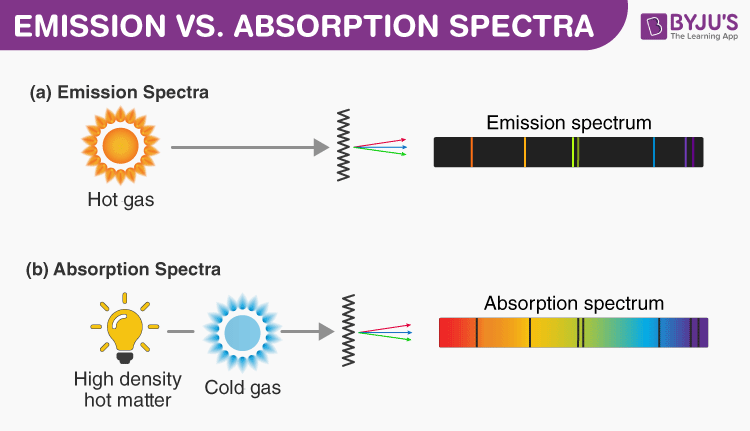
And these are being absorbed (with emphasis on blue). On the other hand, absorbed light is light that isnt seen. Actually, if you just burned hydrogen and looked at its spectra, you would get the Emission Spectra and not the Absorption Spectra, and this Emission Spectra would only show the bunch of blue lines, one purple line, and one red line. All the other colors shown are just part of the natural light being shown down on the element. An absorption spectrum is defined as a spectrum obtained due to electromagnetic radiation transmitted through a gas or any substance. This is the color that will be the opposite of the flame color on the color wheel. Remember, always look at the color area on the rainbow that is blacked out the most. So if blue is being absorbed, the opposite color would be transmitted and this color is orange. An absorption spectrum occurs when light passes through a cold, dilute gas and atoms in the gas absorb at characteristic frequencies since the re-emitted light is unlikely to be emitted in the same direction as the absorbed photon, this gives rise to dark lines (absence of light) in the spectrum. However, there are MORE dark lines in the blue region. If you look at the lines for hydrogen blue, purple, and red are being absorbed. An absorption spectrum is constituted by the frequencies of light transmitted with dark bands when energy is absorbed by the electrons in the ground state to. Therefore, all the other colors would be absorbed.
(This would be orange.) The element hydrogen turns orange when being burned and this color is transmitted to us. This means that if there is a big dark band where blue would be, then the opposite color to blue on the color wheel is being transmitted. Absorption spectroscopy is a spectroscopic technique that measures the absorption of radiation due to its interaction with a sample. You are supposed to look at the dark areas of the absorption spectra and those dark areas indicate that the color which would be there is being absorbed.

I think both the absorption and emission lines are showing which colors are being absorbed.


 0 kommentar(er)
0 kommentar(er)
